|
As OA contains O(16) and N(12) as two front negative sites(Fig.
3b) which are proposed to be important for binding interactions,
an alignment fitting of OA and OA agonists was studied using
rigid superimposition with these two atoms and the corresponding
Cl(m) and N(15) of 29 and CIT as steric
constraints. Similarity comparison of molecules was made using
atom based rigid fit method offered by PowerFit 1.0 from
MicroSimulations. This methodology uses the well-validated SEAL
fitting potential as the objective fitting criteria, and it
utilizes the "global search of best fit" from simulated
annealing [18, 19].
Distance constraints were defined as above, because the QSAR and
molecular modelling results claim the importance of electrostatic
property at m-substituents at benzene in SBO and SBT
compounds. To a certain degree, the fitting graphic results were
similar to what were superimposed by CAChe Visualizer using the
same distant constraints and were then rendered by RASMOL (Fig. 2a and Fig.
2b ), and were displayed in Fig. 4a and Fig. 4b. Molecular
fitting was totally scored by the volume-based steric overlay,
Columbus type electrostatic match, atom-type matching, and
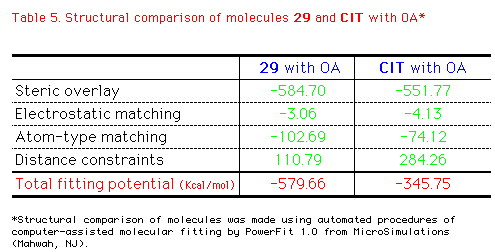
distance constraints as indicated below in Table 5. The
conformation energy was optimized from MM2 force field with bond
stretch, bond angle, dihedral angel, improper torsion, van der
Waals, electrostatic(charge partial), and hydrogen bond. The
final energy of 29, CIT, and OA was -10.6403,
-11.6483, and -10.5948 Kcal/mol, respectively with no remarkable
difference. So the total fitting potential of 29 to
superimpose with OA was -579.66 Kcal/mol totally, while that of CIT
was -345.75Kcal/mol. This result informs that 29 is better
superimposed to OA in similarity means by steric overlay and
atom-type matching. And it's clearly consistant with QSAR results
that although the electrostatic matching of CIT to OA was
with no difference of 29, CIT contains higher
steric hindrances.
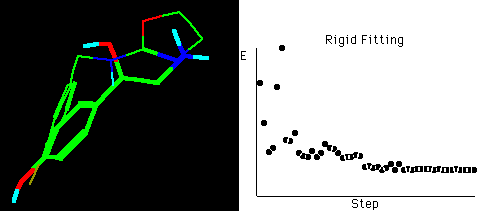
Fig. 4a Molecular rigid fitting of 29 with OA according to
SEAL annealing alignments*
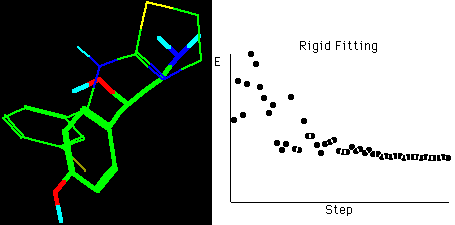
Fig. 4b Molecular rigid fitting of CIT with OA according
to SEAL annealing alignments*
*The right graph shows the fitting simulation process of
optimization in about 55 steps, hydrogens connected to
carbons are omitted for clarity in the structures.
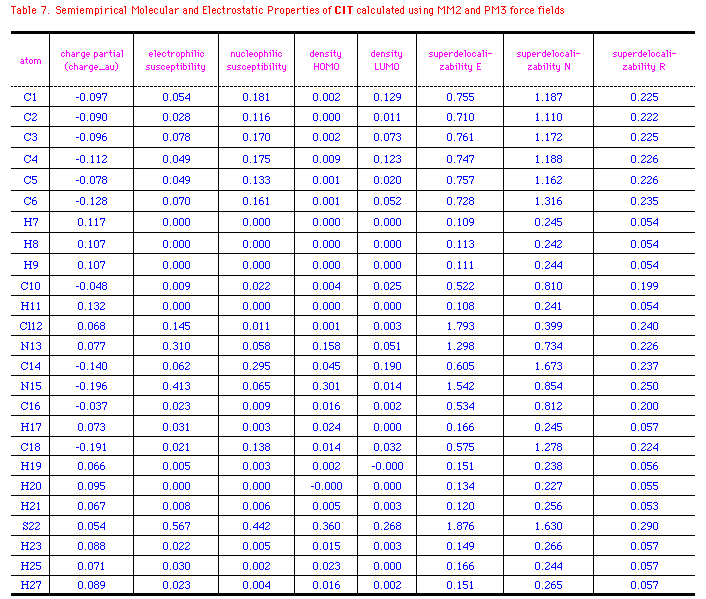
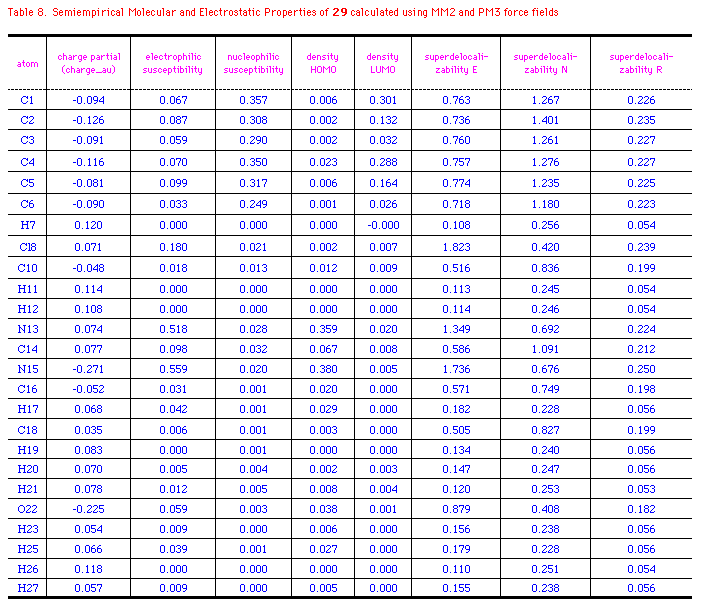
The semiempirical molecular and electrostatic properties of OA, 29
and CIT were further investigated quantitatively using MM2
and PM3 force fields. Table 6-8 listed some electronic
descriptors including charge partial, electrophilic
susceptibility, nucleophilic susceptibility, density of HOMO and
LUMO, superdelocalizability of electrophilic, nucleophilic, and
radical attack at atoms position of OA, 29 and CIT.
The negative charge partial of OA is mainly distributed to
C1(-0.201), C3(-0.183), C5(-0.148), O16(-0.225), and O17(-0.317).
While N12 has a slightly negative electron(-0.038au.), those of
N15 at 29 and CIT are -0.271 and -0.196au.; S22 of CIT
has a positive charge of 0.054au. compared to the negative
oxygen(-0.225) at 29. It's interesting that density of
HOMO in OA relies on C3 and N12, whereas those of 29 and CIT
exits majorly on N15 or S22. It means that these atoms with high
values of HOMO have loosely bound electrons which are reactive to
electrophilic attack. On the other hand, major density of LUMO
disperses on the benzene ring of OA and OA agonists, so the
nucleophilic attack may happen mainly on the aromatic rings. The
electrophilic, nucleophilic susceptibility, and
superdelocalizability investigations make it clear that benzyl
ring of OA and N12, O16, and O17 of the chain are suitable for
either electrophiles, nucleophiles or radicals. N12 of OA and N15
of 29 and CIT are approximately 2-times more active
than the atoms at benzene in accepting an electrophilic attack.
On the contratry, these atoms contain about half the activity of
nucleophilic superdelocalizability of the benzene ring. Besides,
the hetero five-membered ring of 29 and CIT could
be considered aromatic and the data outputed in Table 6-8 support
the idea. Apparently, Cl8(29), Cl12(CIT), and
O16(OA) have the similar behavior in the above mentioned
electrostatic properties, except that Cl8 and Cl16 are more
easily attacked by an electrophile and compose higher
electrophilic susceptibility than O16(OA).
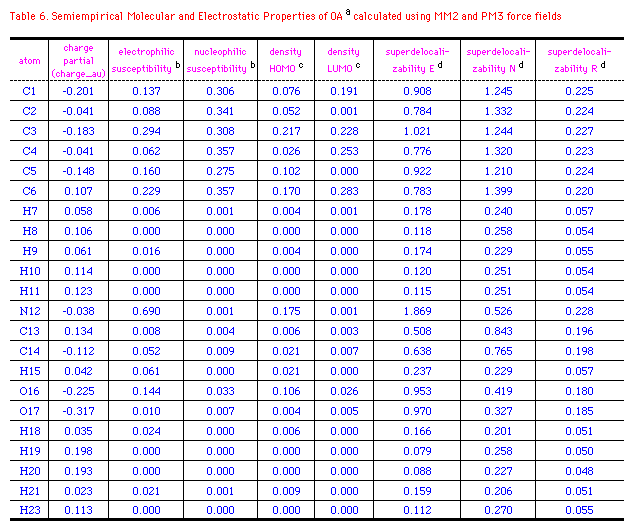
Table6. footnotes
a Ref. structure(Fig.3b)
b Electrophilic and nucleophilic susceptibility
are measures of the susceptibility of the substrate to attack
by an elctrophile or a nucleophile.
c With high values of Density HOMO means loosely
bound electrons which are reactive to electrophilic attack,
with high values of Density LUMO means loosely bound
electrons which are reactive to nucleophilic attack.
d Superdelocalizability E, superdelocalizability
N, superdelocalizability R stand for reactivity by
electrophilic attack, nucleophinic attack, and radical
attack, respectively. They are based on the distribution of
electrons in orbitals and they depend on the reagent energy.
*Ref. structure(Fig.3c) and legend to Table 6.
*Ref. structure(Fig.3a) and legend to Table 6.
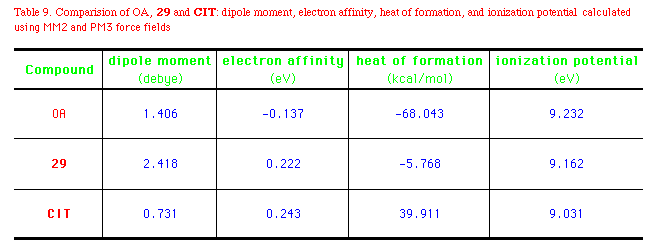
The heat of formations  Hf0 (by semiempirical method
MOPAC) of OA, 29, and CIT differ substantially.
It's well accepted that the global minimum conformation of a
compound(non-receptor bound) can adopt the active(bound)
conformation at a sufficiently low expense of energy. In this
sense, OA and 29 may be speculated to absorb the necessary
energy more easily than CIT as they are in a relatively
low-energy field. The electron affinity of OA is -0.0137eV, while
those of 29 and CIT are about 0.2eV. Besides, OA
seems not likely to penetrate either the cuticle or the central
nervous system(CNS) of insects effectively, since it may be
almost fully ionized at physiological pH. OA and OA agonists of
oxazolines and thiazolines have an ionization potential of
9.0-9.2eV (Table 9). Derivatization of the polar groups would be
one possible solution to this problem in trying to develop
potential pest-control agents. In order to optimize the
activities of these compounds as octopaminergics agonists and
pest-control agents, further detailed and extensive
investigations are in progress. Hf0 (by semiempirical method
MOPAC) of OA, 29, and CIT differ substantially.
It's well accepted that the global minimum conformation of a
compound(non-receptor bound) can adopt the active(bound)
conformation at a sufficiently low expense of energy. In this
sense, OA and 29 may be speculated to absorb the necessary
energy more easily than CIT as they are in a relatively
low-energy field. The electron affinity of OA is -0.0137eV, while
those of 29 and CIT are about 0.2eV. Besides, OA
seems not likely to penetrate either the cuticle or the central
nervous system(CNS) of insects effectively, since it may be
almost fully ionized at physiological pH. OA and OA agonists of
oxazolines and thiazolines have an ionization potential of
9.0-9.2eV (Table 9). Derivatization of the polar groups would be
one possible solution to this problem in trying to develop
potential pest-control agents. In order to optimize the
activities of these compounds as octopaminergics agonists and
pest-control agents, further detailed and extensive
investigations are in progress.
Next
page /Previous page /Table of Contents
|
