|
The molecular electrostatic potential (MEP) is well-known as a
rigorously defined expectation quantity which is measured as the
first-order interaction between he molecular charge (electrons
and nuclei) distribution and a positive unit charge at any point
in space surrounding the molecule. In spite of the time-consuming
computation required by SCF Hartree-Fock wavefunction, the
isopotential maps have proven useful in the analysis of
long-range non-covalent interactions in complex biological
systems, proton affinities, solvation processes, and in the
evaluation of electrostatic charges for molecular mechanics and
dynamics studies[26].
In tabulating the wavefunction data from MOAPC(PM3),
electrostatic potential (ESP) (Fig. 3c) of CIT is
apparently different from that of OA (Fig. 3b). This modelling
agrees with the results from QSAR, in which substituents at m-position
are important for OA agonist-receptor interaction due to their
electronic nature(for a detailed electrostatic data of atoms in
these compounds, see also Table 6-8. and the related contents
there). In Fig. 3 (the magnitude decreases with the spectral
ordering of the colors by white, red, yellow, green, cyan, blue,
and violet charcoal), the electrostatic regions appear consistent
with the QSAR data. The red regions refer to an area where
interactions with negative charges are more favorable. One of
these regions lies to one side of the plane of the benzyl ring
and C(16,18) of 29 and CIT as shown in Fig. 3. In
the case of CIT, the S(22) atom of the hetero-ring is more
positive rather than the negative O(22) of 29. A negative
charge on the molecules in benzyl ring and N(15) of oxazolines
and thiazolines would be favorable to hydrogen bond donation. The
blue regions interact extensively with a positive charge and thus
would be an area to suspect hydrogen bond acceptor groups on the
protein.
Fig. 3. Continuous electrostatic potential mapped onto electron
density surface of a) 29, b) OA, and c) CIT.
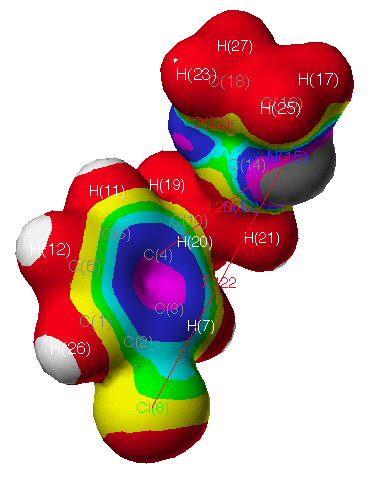
Fig. 3a Continuous electrostatic potential mapped onto electron
density surface of 29
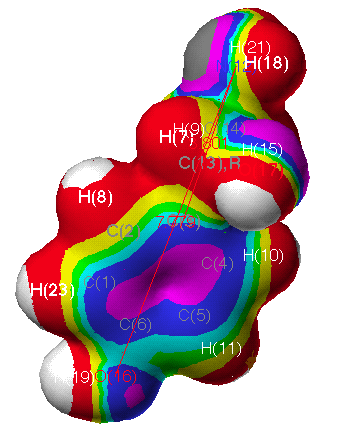
Fig. 3b Continuous electrostatic potential mapped onto electron
density surface of OA
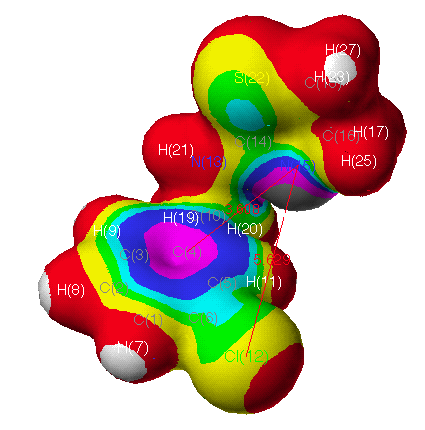
Fig. 3c Continuous electrostatic potential mapped onto electron
density surface of CIT
Next
page /Previous page /Table of Contents
|
