|
Cyclization of Polyalkene-1,1-dicarbonitriles Upon Direct Excitation
and Photoinduced Electron Transfer to a Pyrylium Salt
Max-Planck-Institut für Strahlenchemie, P.O. Box 101365,
D-45413 Mülheim an der Ruhr, Germany
1. Introduction.
Photoinduced electron transfer from isoprenoid polyalkenes to the excited state of
suitable electron acceptors in the presence of nucleophilic solvents has proven as an
intriguing approach to initiate cascades of regioselective functionalization and
subsequent radical cyclizations en route to new mono- and polycyclic compounds
[1-6]. Among the polyalkenes examined, 1,1-dicarbonitriles such as 1, 2 and 3
constitute a class of special interest. Both direct irradiation of these readily available
compounds, as well as electron transfer to acceptors absorbing at higher wavelengths,
namely 2,4,6-triphenylpyrylium tetrafluoroborate (P+BF4-),
give rise to synthetically useful products in high yields.
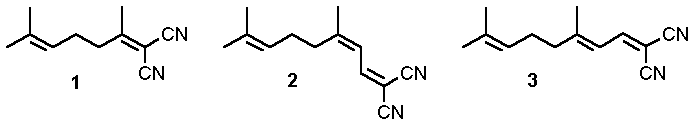
Fig.1 Polyalkene-1,1-dicarbonitriles
|
