|
 
B.M. Aveline and R.W. Redmond
Exclusive Free Radical Mechanisms of Cellular Photosensitization
1. Introduction
Over the last decade, there has been increasing interest in
photosensitization mechanisms in biological systems, in relation to both deleterious and
therapeutic aspects of this phenomenon [1,2] . Photosensitization reactions are generally
considered as belonging to either the type I (radical mediated) or type II (singlet oxygen
mediated) definition [3] . Most photosensitizers used in photodynamic therapy (PDT)
(e.g., porphyrins, chlorins or phthalocyanines) display significant production of the
long-lived triplet excited state, which generally undergoes facile energy transfer to
molecular oxygen, leading to the formation of singlet oxygen. For a type I reaction to
take place, a radical-generating reaction must compete with energy transfer to oxygen.
However, even in cases where type I reactions may occur for these compounds, the type II
reaction will usually take place in tandem and it is difficult to differentiate the
photobiological effects which are exclusively due to radical species.
Although experimentally one is still confronted with the problem of how to specifically
generate free radicals, the study of radical-induced damage in living systems has become a
topic of great interest in biology and medicine [1,2]. Attention has been increasingly
focused on the role of free radicals in normal physiological conditions (such as the
so-called "respiratory burst" [4], important in the human host defense) as well
as in various pathologies (e.g., cancer [5,6], inflammation [7], reperfusion injury [8],
ocular damage [9,10], aging [11,12] or drug photosensitivity [13]). Radical reactions are
also known to mediate biochemical phenomena at the cellular level such as lipid
peroxidation [14], protein damage [15] and strand-breaks in DNA [16].
We have been working towards the development of photolytic radical generating molecules,
to facilitate the study of pure radical effects in biological systems, where the free
radical species are produced in a selective manner without complications arising from
concomitant formation of long-lived triplet states via which type II photosensitization
may occur. In this context, we have recently investigated the photochemistry of
thiohydroxamic esters by 355 nm laser flash photolysis [17]. The primary photoprocess
undergone by these molecules, upon pulsed excitation, is a homolytic nitrogen-oxygen bond
cleavage which can be summarized by reaction (1).
N-O bond homolysis in these ester derivatives leads to simultaneous formation of the
2-pyridylthiyl radical (PyS) and RCOO and RCOO , an acyloxy
radical. In the case of compounds 2a, 2b and 2c (see Figure 1),
decarboxylation occurs rapidly (<10 ns) to yield primary, secondary and tertiary carbon-centered radicals, respectively, in addition to pys , an acyloxy
radical. In the case of compounds 2a, 2b and 2c (see Figure 1),
decarboxylation occurs rapidly (<10 ns) to yield primary, secondary and tertiary carbon-centered radicals, respectively, in addition to pys . On the other hand, irradiation of the aroyl derivative 2d
produces C6H5COO . On the other hand, irradiation of the aroyl derivative 2d
produces C6H5COO , an oxygen-centered radical,
which decarboxylates with a much lower rate [18] (tau = 310 ns in acetonitrile [17]) and
can therefore participate in subsequent reactions before decarboxylation takes place. , an oxygen-centered radical,
which decarboxylates with a much lower rate [18] (tau = 310 ns in acetonitrile [17]) and
can therefore participate in subsequent reactions before decarboxylation takes place.
The parent compound, N-hydroxypyridine-2(1H)-thione, was also observed to undergo
efficient homolytic N-O bond cleavage upon pulsed excitation. As shown by equation (2),
this process gives rise to the formation of PyS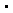 and the hydroxyl
radical, and the hydroxyl
radical, 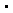 OH, one of the most powerful oxidant species known. OH, one of the most powerful oxidant species known.
However, an extensive investigation of the photochemistry of compound 1 by laser
flash photolysis demonstrated that this molecule cannot be considered as a clean
photochemical source of hydroxyl radicals for the selective study of 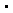 OH
reactions in biological systems since it also undergoes other primary photoprocesses,
which produce potentially toxic species [19,20]. OH
reactions in biological systems since it also undergoes other primary photoprocesses,
which produce potentially toxic species [19,20].
In this work, photolytic, selective (compounds 2a-2d) and non-selective (compound 1)
radical generators have been applied to cellular systems (murine L1210 lymphocytic
leukemia cells). Our main goal was to investigate the effects of unambiguous radical
photosensitization in biological systems. In order to provide some insight into their
phototoxicity reaction mechanisms, thiopyridones have also been studied for their ability
to initiate lipid peroxidation and to induce apoptosis. This report provides evidence that
exposure of murine leukemia cells to UVA light (355 nm) in the presence of thiopyridone
derivatives, triggers biological damage, which can be exclusively attributed to free
radicals and constitutes a first step toward the comparison of the relative
bioreactivities of different radical types (sulfur-, carbon- and oxygen-centered radicals)
and singlet oxygen in cells.
|
